The Sun is a pretty average star in
our Galaxy, one of several X 1011 . It is
situated out in the disk of the Galaxy, an object that is about
105 light-years (or about 6 billion AU) across:

The Sun is important for our
planetary system in a number of ways:
In the US, we mostly use the Fahrenheit scale, but it is an antique relic like the English measurement system. All other countries use the metric temperature scale (Centigrade or Celsius).
At 1 bar pressure, water boils at 100 C (212 F) and freezes at 0 C (32 F). A hot Tucson day in the summer usually reaches close to or slightly above 40 C, while a nice winter day like now reaches a maximum temperature of about 20 C. It's about 20 C in this lecture room.
Here's the conversion formula:
T (in C) = [T (in F) - 32 ] X [100/180]
Interestingly, 40 below zero Fahrenheit is also -40 C.
Planetary scientists prefer a temperature scale that has no negative values, so they frequently use the Kelvin scale, which measures temperature from absolute zero. Absolute zero is the temperature at which any body will not yield any heat by any process. In other words, that's as cold as anything can ever get. Absolute zero turns out to be -273 C. So,
T (in K) = T (in C) + 273
Room temperature (that is, in this room) is about 293 K, or say 300 K in round numbers. Remember this number.
In the farthest reaches of intergalactic space, it never gets colder than about 3 K.
The temperature at the visible surface of the Sun is 5800 K. That's hot enough to vaporize any material, even rocks and stainless steel!
Solar Radiation
The Sun radiates energy into the solar system mostly by photons. At its surface temperature of 5800 K, most photons have wavelength between 0.7 and 0.4 micrometers (visible), and the Sun looks white. It takes each photon about 500 seconds to reach the Earth after it is emitted from the Sun's surface.
The solar constant is the power that a 1-meter square surface receives from the Sun when it is pointed directly at the Sun, at a distance of 1 AU from the Sun.
Solar constant = 1368 Watts = 1368 Joules/second = 1368 X 107 ergs/second
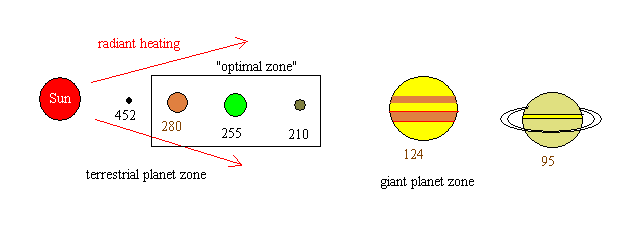
When planets absorb photons, their surfaces heat up. Here is what we'd predict for the surface temperatures of planets at various distances from the Sun:
Spacecraft
are subject to these same laws, so the temperature-sensitive
parts of spacecraft must be carefully packaged to keep them in a
specified temperature range (actually very similar to the range
that humans prefer).




Structure of the Sun
The Sun is classified as a G2 dwarf
star ( a very ordinary type of star, and a type of star that we
now know is fairly likely to harbor planets). The Sun
formed from an interstellar cloud 4.5 Gyrs ago, out of material
that had been at least partially recycled from earlier
generations of stars in our Galaxy. Almost all of the
elements in the Sun beyond element number 2 (helium) were made
in these earlier generations of stars.
Here is a cross-section of the Sun, as determined from solar seismology, or helioseismology:
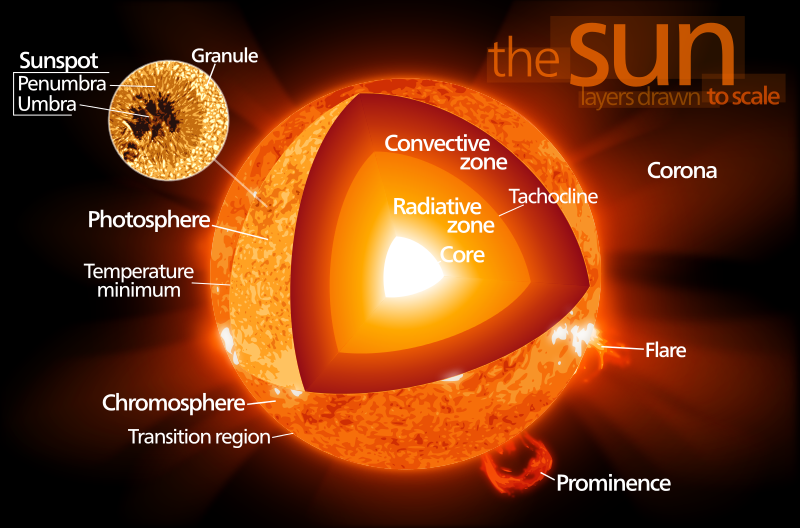
The Sun's luminosity comes from thermonuclear energy. When a plasma of hydrogen is heated to around one million K (106 K) or hotter, the kinetic energy of the protons is high enough for the protons to fuse to form the hydrogen isotope deuterium, and the helium isotopes helium-3 and helium-4. Gamma rays (high-energy photons) are emitted in the process, and they heat the plasma. Thermonuclear fusion does not occur in any planet (they're not hot enough), but it can occur in artificial devices such as hydrogen bombs.
As a result of the fusion of protons, the Sun's center is gradually being converted to mostly helium. However, the Sun's outer layers still have much the same composition as the material out of which the Sun formed.
Here is a comparison of the sizes of
the Sun and the Earth. The Sun's diameter is about 100
times the Earth's:

One of our main goals in this course is to understand how the solar abundances of elements, the raw material of the so-called solar nebula out of which planets formed (see artist's conception below), got transformed into the planets we see today.

The disk of gas and dust from which
the Sun and planets formed, 4.5 Gyrs ago, also called the solar nebula.
Abundances of elements in the Sun's atmosphere
Absorption lines in the sunlight at specific frequencies tell us about the composition of the Sun's atmosphere. Here is a table of the relative number of atoms in the Sun's atmosphere, relative to a million atoms of silicon. That is, if we had a sample of solar material that contained 106 atoms of Si, it would contain the following numbers of other atoms:
If we plot this on a "pie" diagram, it looks like this:
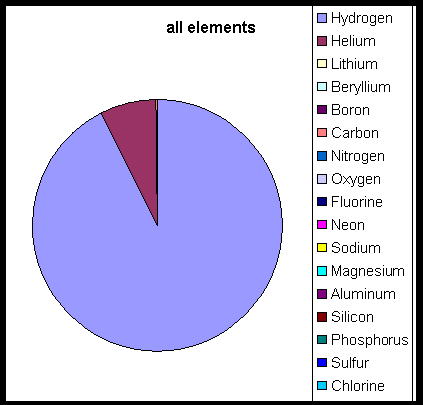
That is, it's almost all hydrogen and helium.
Here is a view of the abundances on a logarithm scale (log 109 = 9):
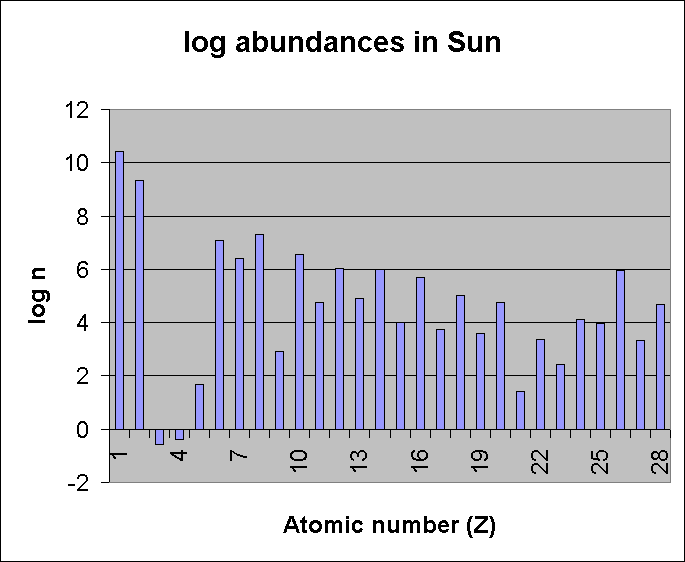
On this diagram, notice the
depletion of elements 3, 4, 5 (Li, Be, B). These are not
so depleted in the "solar-nebula" material, but they are
strongly depleted in the Sun due to hermonuclear reactions with
protons. Also notice the alternation of odd and even
elements. The process of nucleosynthesis in stars
has produced virtually all the elements beyond H and He, and the
tendency to produce more even elements than odd
elements is a reflection of thermonuclear reaction processes in
stars. We see this even-odd alternation in elemental
abundances in planets as well as in the Sun, although not so
strongly.
We have another method to find out
the composition of the original solar nebula: the composition of
the most primitive meteorites. These represent the
composition of the dust component in the solar nebula.
They are depleted in the most volatile elements (see Homework
#2).
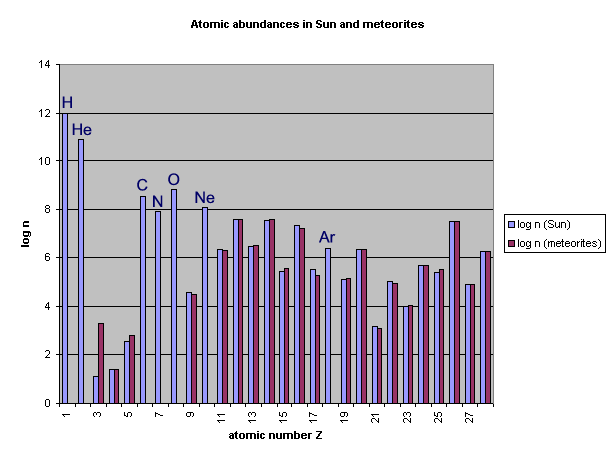
Much later in the course, we'll talk
about meteorites.
Iron is the last element to be built up by fusion processes in stars. Every element beyond iron costs more energy to make than it liberates. The elements beyond iron are made in supernova explosions under conditions of high neutron densities. Many radioactive isotopes are created in these same conditions.
Now if we plot another "pie" diagram of everything left after hydrogen and helium, here's what we get:
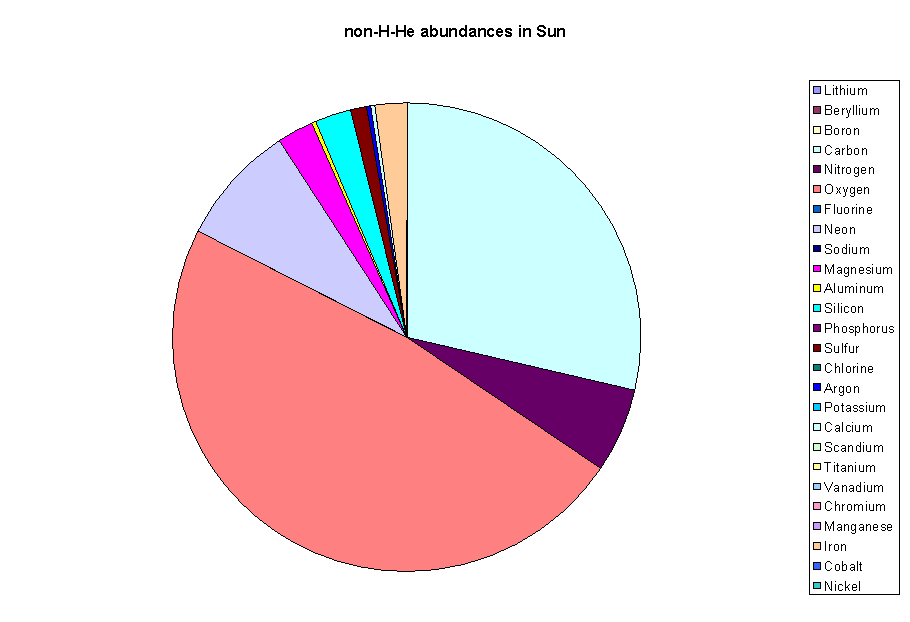
If the solar system formed out of
this kind of material, this is what we'd use to make
planets. Notice that the number of atoms of "volatile"
elements is much higher than the number of atoms of
"not-volatile" elements. Among the latter, notice that the
main ones are Mg, Al, Si, S, Ca, and Fe. These combine
with each other and with O to form molecules which are the main
constituents of "rock".
Fe (iron) is much denser than the other components in rock, and tends to separate out when it is melted. The elements which dissolve easily in molten iron and thus tend to be carried away along with it are called siderophile (iron-loving) elements. These include Ni (nickel), Co (cobalt), Pt (platinum), Au (gold), and Ir (iridium). These elements will tell us important things about the Earth and Moon later in this course.
It is also important to realize that life had to form out of this same mixture of elements. What are the main elements that go into the molecules of living matter? Here is a diagram of a molecule of DNA -- the basic building block of life: